
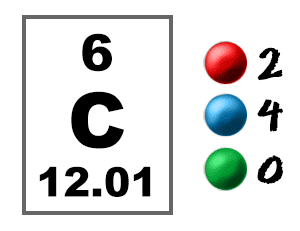

CARBON ELECTRON CONFIGURATION CONDENSED FULL
They want us to write the electron configuration. To write condensed electron configurations (also called abbreviated electron configurations) Carbon (C) we first write the full electron configuration for th. Unless they say full ground state electron configuration, we usually just assume that this is the method. Well put six in the 2p orbital and then put the next two. The lowest-energy distribution of electrons in the sublevels for an atom of a particular element is called the state electron configuration for that element. The principal quantum number and the number of electrons are always specified. The p orbital can hold up to six electrons. Reason: The only acceptable electron configuration for hydrogen is 1s1. The next six electrons will go in the 2p orbital.
Since 1s can only hold two electrons the next 2 electrons for Argon go in the 2s orbital. This will be the primary method to write electron configurations because it's the faster, easier way to do it. In writing the electron configuration for Argon the first two electrons will go in the 1s orbital. It's important to know which elements are we being asked to find the electron configuration off? And what's the noble gas before it? We're going to say, moving forward. Learning Objectives Give the electron configuration for an atom using Bohr’s model, box orbital diagrams, and quantum mechanical notation. We have our d block here and we have our f block down here with the condensed electron configuration. Carbon atoms occupy the upper-back-left, upper-front-right subcubes, the lower-front-left and lower-back-right subcubes with the bond length being 15.4 nm.The. We have our s blank where begins with one s. For example, the ground-state configuration for carbon is: He. And if we take a look here, remember, this is our reimagining of the periodic table. Transcribed image text: Using condensed notation, write the ground-state electron configuration of this element In your answer, use square brackets around the noble-gas core, insert one space between subshells, and do not worry about superscripts. We start at the last noble gas before the desired element. We're going to say here that the condensed electron configuration is a faster way to write out electron arrangements for elements or ions were going to say with condensed electron configurations. Transcribed Image Text: Part F - Choose the correct condensed electron configuration for: the 1+ cation whose corresponding neutral has the following condensed electron configuration: neutral - Kr5s24d105p1 O Kr5s4d105p1 O Kr5s4d10 O Kr5s4d105p1 O Kr5s☤d75p3 Submit Request Answer.


 0 kommentar(er)
0 kommentar(er)
